Children in 2-18 age group will get 2 doses of the Bharat Biotech vaccine from pre-filled syringe
NEW DELHI:
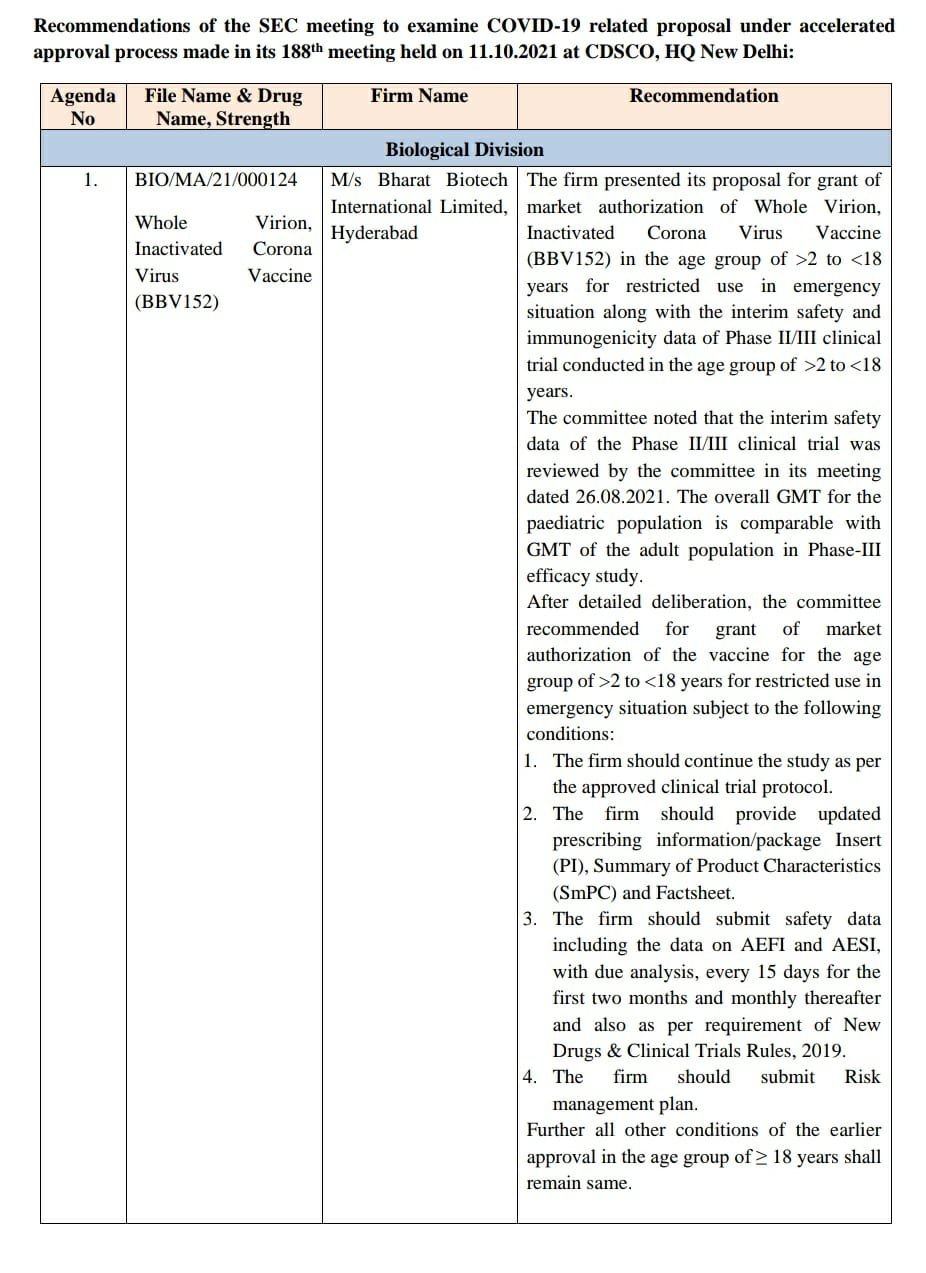
In a major development, the much-awaited nod for paediatric Covid-19 vaccination has come, with the Subject Expert Committee (SEC) of the Central Drugs Standard Control Organization according approval to Bharat Biotech’s paediatric Covid-19 vaccine on Tuesday.
This will be a pre-filled syringe to be used for children. Covaxin and other Covid-19 vaccines for adults are available in multi-dose vials.
Kids’ trials over
As per sources, the drug regulator had given its nod to Bharat Biotech to conduct trials on children in May this year, which were completed in September. In the trials, Covaxin was administered to 525 children in the 2-18 age group.
The vaccine dosage for children will be 0.5 ml, the same as for adults. However, when administering vaccines to children, the accuracy of the dose is crucial.
Administering vaccines from vials to syringes can at times be inaccurate, resulting in the dose being less or more than the recommended 0.5ml.
Children will receive two doses of the vaccine. For adults, the government recommends a gap of 4-6 weeks between two shots.
Conditions apply
After detailed deliberation, the SEC recommended the grant of market authorization of the vaccine for the 2-18 age group for restricted use in emergency situations, subject to the following conditions:
• The firm should continue the study as per the approved clinical trial protocol.
• The firm should provide updated prescribing information/package insert (PI), summary of product characteristics (SmPC) and factsheet.
• The firm should submit safety data, including data on AEFI and AESI, with due analysis, every 15 days for the first two months and monthly thereafter and also as per the requirements of the New Drugs & Clinical Trials Rules, 2019.
• The firm should submit a risk management plan.
• Further, all other conditions of the earlier approval in the age group of ≥ 18 years shall remain the same.






